Jose L. Agraz, PhD
para-Hydrogen Induced Polarization (PHIP)PHIP : Introduction
Abstract
Existing para-hydrogen induced polarization (PHIP) instrumentation relies on four factors to hyperpolarize endogenous substances: a static magnetic field (Bo), temperature, pressure, and software sample control. These hyperpolarized sub- stances have enhanced magnetic resonance imaging (MRI) signals over 1000 fold, allowing for molecular imaging. We discuss the improvements in Bo generation, sample heating, sample manipulation, and software sample control to produce polarized HEP. We show that HEP polarization doubles from previous efforts by use of: 1) A voltage controlled voltage source, 2) A modified pinch valve, and 3) LabView software sample control developed in our laboratory
Introduction
Cancer, Alzheimer’s, heart disease, and stroke are the leading causes of death and debilitation among Americans. These diseases are poorly diagnosed at early stages and linked with considerable morbidity and mortality. The current gold standard for muscle ischemia, cancer, and Alzheimer’s disease screening varies from a simple self exam (breast cancer), to troponin blood level test (Heart ischemia), to Computed Tomography (CT) scan (ischemia, cancer, Alzheimer’s disease), and the more invasive needle and excisional biopsies. These methods have worked in the past, but as the understanding of disease expands, these methods are becoming obsolete for earlier disease detection. Recent studies had shown that the understanding of cell signaling or management of basic cellular activities, in conjunction with advances in magnetic resonance imaging (MRI), may lead to the diagnosis, treatment, and in some cases the prevention of disease. Cancers such as paraganglioma and pheochromocytoma (SDH), leiomyoma, and certain cases of renal cell carcinoma are linked to the abnormal accumulation of succinate and fumarate in cells, corresponding to aberrations in the Kreb’s cycle. Similarly, high levels of succinate in a failing heart have been shown, but the quantification of succinate process mechanism and the key to heart disease is still unknown. Furthermore, the earliest sign of Alzheimer’s disease (AD) is the reduction of cerebral glucose. Studies have suggested that the metabolism of glucose is shifted (among other mechanisms) to the activation of the gamma-aminobutyric acid (GABA) as alternate source of energy. This sort of energy leads to the brain digesting itself. Ensuing studies have also shown mitochondrial abnormalities in AD patients. Equivalent to mechanism enabling aberrations in the TCA and high levels of succinate in failing heart quantification, the energy consumption mechanism of an AD brain change still eludes researchers. Thus, transforming these metabolites such as, succinate and GABA, into magnetic resonance imaging (MRI) contrasting agents, through the hyperpolarization method, is a promising, safe, and minimally invasive method for disease diagnosis to the cellular level.
Cell Pathways, the Key to Early Disease Detection
The Kreb’s cycle, also known as the tricarboxylic acid (TCA) cycle, is a basic cell metabolic mechanism that employs a series of chemical reactions to generate energy in the form of adenosine triphosphate (ATP) through oxidation of biological molecules. This series of chemical reactions, also known as pathways, modify an initial molecule to form another product, to either be used in the beginning of other pathways, as intermediates to a pathway, or stored in the cell. Cancers such as paraganglioma and pheochromocytoma (SDH), leiomyoma, and certain cases of renal cell carcinoma are linked to the abnormal accumulation of succinate and fumarate. Succinate and fumarate are intermediates substances in the Krebs cycle and abnormal accumulation of these intermediates indicates aberrations in this cycle.
The MRI signal
The method of contrast agents hyperpolarization arose from the need of overcoming
the poor signal-to-noise ratio (SNR) inherent to MRI. Poor signal is due to
the low spin polarization at thermal equilibrium. Thermal equilibrium
is when the alignment of spins within a magnetic field in either of two orientations,
parallel or anti-parallel, and determined by the Boltzmann distribution.
Thermal equilibrium polarization is defined as the difference in spin populations (Pth)
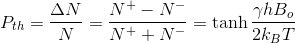
MR signal intensity is proportional to polarization so the lower sensitivity
From the Boltzmann distribution or polarization
increasing MR sensitivity can be accomplished in two ways, decreasing
temperature and/or increasing the magnetic field. However, decreasing temperature
for in vivo imaging is not feasible without harming the subject and increasing
the magnetic field brings a new set of issues to overcome, such as cost and subject
safety. Hyperpolarization though, is independent of magnetic field strength
and temperature. The hyperpolarization of molecules creates an artificial non-
equilibrium distribution of excitable nuclei, increasing signal strength at multiple
orders of magnitude and allowing ample signal (signal to noise ratio, SNR) for
cell metabolites real-time imaging. In fact, hyperpolarization does not affect the
signal intensity of water protons. Instead, hyperpolarized molecules become a
source of signal themselves. A slightly larger proportion of spin adopts
the parallel state (lower energy) and can be excited into an anti-parallel state
(at body temperature, 5ppm for 1H and 1 ppm for 13C,
providing the basis for
MRI. Unfortunately, at thermal equilibrium, a small number of
excitable spins limits MRI to mostly tissue structure imaging. However,
researchers have met some success increasing SNR and the ability to image beyond
tissue structure. For example:
- Gadolinium based agents, improving T1 contrast and repetition rate. Toxic in large amounts
- Increase of main Magnetic Field Bo, increases the number of excitable spins. Expensive at $1M/Tesla
- Parallel imaging, multiple receiver coils, increases SNR several fold. Complicated technique and computation intensive.
- Cooling receiver electronics to ∼10K, decreases thermal noise, thus SNR. Complicated and expensive.
Hyperpolarization
The efforts to overcome thermal equilibrium leading to signal enhancement in Nuclear
Magnetic Resonance (NMR) began as early as the 1930’s with crude experiments
on paramagnetic materials. This was followed by experiments on relaxation
times with Cr and Ti Alum, effectively demonstrae that relaxation times could be
increased in a constant magnetic field, independent of the field’s direction.
Finally, hyperpolarization came to life in 1953 with the theoretical discovery of
spin transfer from one nuclei to another through cross-relaxation effect described
as the Overhauser s effect. Then, the dynamic nuclear polarization (DNP)
method was demonstrated in 1954 with experiments in magnetic susceptibility using
lithium. DNP research continued to evolve through the 1960’s leading
to the discovery of the chemically induced dynamic nuclear polarization (CIDNP)
method. This method was reproduced by the disproportionation of radical pairs.
Subsequently, Weitekamp’s research work on J-coupling measurement techniques in [1-13C]
Benzene in the early 1980’s, led to the PASADENA (parahydrogen and synthesis allow dramatically enhanced
nuclear alignment) method and the basis for PHIP.
PASADENA was first proposed in 1986 and demonstrated in 1987 by hyperpolarizing propionitrile
[CH 3 CH 2 CN], resulting in a 100-200 times signal increase. Interestingly
and unknown to most, PASADENA was first observed, but misinterpreted, by
Seidler in 1983.
The PASADENA method is described as a proton signal enhancement resulting from the
breakdown (aided by a catalyst) and pairwise transfer of
p-H2 during substrate hydrogenation. Hydrogenation occurs at magnetically
inequivalent sites on the substrate molecule within a high magnetic field.
Concurrently, the ALTADENA (adiabatic longitudinal transport after dissociation
engenders net alignment) method, an adiabatically
transfer of spin at a low magnetic field, surfaced in 1988 demonstrating hyperpolarization
of ethylbenzene within a weak magnetic field. PASADENA
and ALTADENA are similar methods of hyperpolarization, but differ in the following; the
PASADENA method results in a spectra with anti-phase multiplets of
reaction products and introduces the fast breaking of magnetic equivalence as the
hydrogenation is taken place at a high magnetic field, while ALTADENA takes
place at a low magnetic field, followed by an adiabatic transfer of the hydrogenated
product to the high magnetic field. However, heteronuclerar hyperpolarization,
the spin transfer from p-H2 to other nuclei, was not demonstrated until
1987, when a new method emerged describing the monitoring of reaction products
and the potential for much greater sensitivity. Thereafter, this
method was known as PHIP.
Further PHIP work was published in the early 1990’s demonstrating the optimization
of spin transfer using the insensitive nuclei enhanced by polarization
transfer (INEPT) sequence. In this work, the hyperpolarization of other low γ
nuclei such as; 29Si, 15N, 17O, 109Rh was
proposed and later demonstrated. Then, slightly more sophisticated sequences such as; the Gold-man’s
& Hakee’s sequences were demonstrated in vivo with mild success in 1996
& 2001. More recently, the Kadlecek sequence had shown great
simulation results, but the sequence is yet to be tested in vivo.
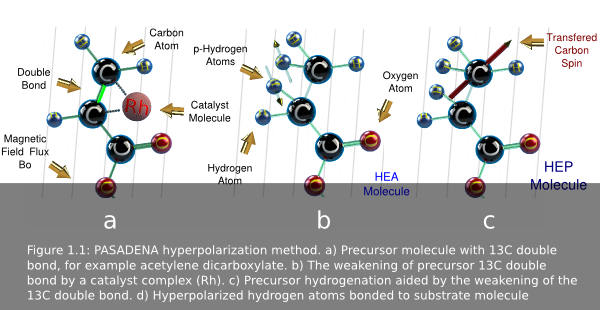
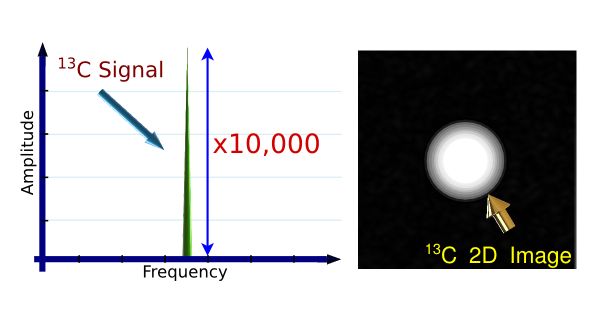
The PHIP method concept is based in the chemical addition of p-H2 to a substrate
or precursor molecule (gas or liquid)(fig 1.1a). This precursor is pre-loaded
with a Rh catalyst complex that weakens the 13C double bond. The
mixing of p-H2 and the precursor occurs in a reaction chamber where a
catalyst assisted hydrogenation reaction takes place, adding the two p-H 2 atoms
across the carbon-carbon double bond. The catalyst adds the hydrogen
in a concerted reaction maintaining the spin orientation. Then, the Rh complex
dislodges. The spin alignment then transfers from the added p-H 2 atoms to
13C in the newly created molecule by irradiating the mix with RF.
This method results in a signal enhancement of 10,000 or more.
Nowadays, PHIP research is reaching new limits at Cedars-Sinai Medical center
with the development of a new and more stable, sterile, and dependable PHIP
design prototype. This new development marks the firsts steps towards a clinical
Limitations of MRI
MRI has contributed greatly to the diagnosis and understanding of many diseases. The modality offers great soft tissue contrast, high spatial resolution, unlimited repeated measurements, and is non-invasive. More specifically, MRI only allows the imaging of protons in water molecules and fats. Thus, cell metabolism imaging has yet to be fully achieved using MRI. This type of imaging requires a large SNR, but MRI is restricted by in vivo restraints, namely low concentration of excitable hydrogen atoms, dielectric losses, and high costs.
Significance of PHIP Methods
Human body anatomy and cell signaling pathways have already been explored in MRI. However, because observable molecules are very small (order of 10 −5 ), poor sensitivity hinders imaging metabolic processes, and further developments have reached a plateau. Increasing the magnetic field strength provides a solution to MRI sensitivity, but at an expense of $1M/Tesla. Other technical issues include: power deposition, magnetic field homogeneity, and overall safety. PHIP instrumentation offers an inexpensive solution to this problem. PHIP is capable of bringing five orders of magnitude more signal than proton imaging, while also providing an insight into nanochemistry, metabolics, and faster imaging, with virtually no signal background noise due to the low natural abundance of 13C, 15N, 31P.
PHIP Method
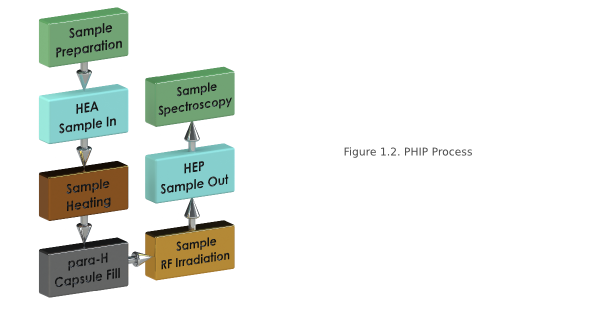
PHIP is our target hyperpolarization method. The polarization process takes place in a low Bo field as follows: The precursor (for example fumaric acid) is preloaded with a Rh-complex as a catalyst, effectively weakening the carbon double bond. The p-H2 is then mixed with the precursor in the reaction chamber which is kept at temperature (40-60C) and pressure (2-7 bar). Once the mixture is in the chamber, hydrogenation takes place. Here, two hydrogen atoms are chemically added across the carbon double bond. The result is the target product (for example succinic acid). The transfer of spin, or hyperpolarization, from the two attached hydrogen atoms to the 13C atom in product happens when the product is exposed to a RF sequence (for example, the Goldman Sequence) enabling the polarization transfer. The product is then delivered intravenously for in vivo imaging. In relation to the other techniques, the PHIP method is cheap, an easier to implement, and continuous flow is possible. However, there is a limited number of candidate molecules, and the filtering of the toxic Rh catalyst complex, both difficult problems to overcome

 Introduction
Introduction
 Software
Software
 Bo Control
Bo Control
 Bo Noise
Bo Noise
 Pinch Valves
Pinch Valves
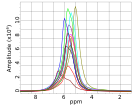 Conclusions
Conclusions
Feb 14th, 2015 at 5:09 pm